Dear Pediatric Provider,
We hope the information and resources posted on this page have been helpful. We will continue to share what we learn as a Pediatric System of Care (PSOC) in response to the pandemic to ensure we optimize our community’s response to this and future challenges.
If there’s information you would like to share within this collaborative of almost 350 pediatric providers, please complete the form in the Questions and Comments section below.
This PSOC we will connect each of us as a stronger, more informed and responsive team of child health professionals and institutions working together to address the challenges facing children today and into the future.
Thank you for joining our Pediatric System of Care.
Northeast Florida Pediatric Society, Wolfson Children's Hospital, Partnership for Child Health
On this page, pediatric healthcare providers will receive the LATEST INFORMATION AND RESOURCES on COVID-19 to help manage patients and families. |
CLICK ON THE + BELOW for topics and resources. SEND YOUR QUESTION in the comment box below to receive answers from our pediatric team. Answers will be researched and published here. |
EMAILS WILL BE SENT TO YOU as new information becomes available. |
COVID Pediatric Testing Sites in Jacksonville
There is a gap in knowledge in the community regarding testing for pediatric clients. Please help by sharing this information. This email provides information on Department of Health (DOH) as well as City of Jacksonville COVID-19 testing sites. Each site provides testing for the pediatric population. Parents can make an appointment with DOH by calling 904-253-1850 or they can utilize the walk up testing sites listed below. There is rarely a wait with walk up testing. Updates to the schedule are provided on our website (http://duval.floridahealth.gov/) and through the city's website at coj.net. In addition, DOH offers in-home COVID testing for those that are home-bound. Parents should call the aforementioned number and make an appointment for such services.
DOH Testing Sites- Scheduled tests can be done by calling 253-1850. Testing done at Central Health Plaza
-
- Oral swabs
- Serology testing available
- Walk-up testing at Central Health Plaza
-
- Monday – Friday 9:00a – 3:00p
- Oral swabs
- Those that are disabled can remain in their car
- Mobile Unit
-
- 7/20 – 7/31 at River City Walmart on the northside 9:00a – 3:00p
- Will announce sites as they come available
- Oral swabs
Below are the City contracted COVID testing sites that opened on Monday, July 20, 2020 from 11am to 7pm for the next 6 months. Logistics Health will be able to test 192/day and be operational Monday through Friday each week.
- Oceanway Senior Center
12215 Sago Ave. W
Jacksonville, Florida 32218 - Lane Wiley Senior Center
6710 Wiley Road
Jacksonville, Florida 32210 - Leroy D. Clemons Senior Center
55 N. Jackson Avenue
Jacksonville, Florida 32229
Thank you,
Pauline J. Rolle, MD, MPH, CPH
Interim Health Officer
Medical Executive Director
DOH-Duval
In the last two months, Pediatric Multisystem Inflammatory Syndrome associated with COVID-19 has been diagnosed in a few children. Here is a research and management document from the Royal College of Paediatrics and Child Health on the rare syndrome. View PDF
Families involved with child welfare may have additional stresses as a result of the pandemic. Here is a Q and A from the Children’s Bureau where you can Learn more guidance to child welfare agencies and courts.
The OECD (Organization of Economic Cooperation and Development) provides excellent overview of the impact of COVID-19 on children from a global perspective. View PDF
Policy Brief: The Impact of COVID-19 on Children by UNICEF
April 3, 2020 CDC recommends wearing cloth face coverings in public settings where other social distancing measures are difficult to maintain (e.g., grocery stores and pharmacies) especially in areas of significant community-based transmission.
https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover.html
An examination of pediatric coronavirus cases in China shows the potential effect on kids. Cases of COVID-19 among children in China have been less severe than those in adults, according to a new study. In this study, “Epidemiology of COVID-19 Among Children in China,” published in the journal Pediatrics, researchers examined 2,143 infected children under the age of 18 in China that were reported to the Chinese Centers for Disease Control and Prevention as of February 8.
About two-thirds of the children had suspected cases of COVID-19, and the rest of the cases were laboratory-confirmed. There were no major differences in the number of boys and girls.
According the American Academy of Pediatrics, other findings included:
- About half of the children had mild symptoms: fever, fatigue, cough, congestion and possibly nausea or diarrhea
- More than one-third were moderately sick, with additional symptoms including pneumonia or lung problems
- About 4% of the children had no symptoms
- 6% of the children developed serious illnesses, compared to up to 20% in adults
- One 14-year old by who tested positive died, and 13 children were considered in critical condition
- The study also found infants had higher rates of serious illness than older children. Less than 11% of children had severe or critical cases compared to 7% of children ages 1 to 5, 4% of children ages 6-10, 4% of children ages 11 to 15, and 3% of children 16 and older
In the study, the authors proposed several possible reasons for the difference of the severity of illness between kids and adults, including children having fewer opportunities for exposure, higher levels of antibodies against viruses or different response from their developing immune system. The virus also may not bind as well to children’s cells.
The study was limited by a short window of time and a high percentage of severe and critical cases without laboratory confirmation. Those cases potentially could have been other respiratory infections.
Source: Children’s Hospital Association
| The AAP has recently published a new report, "Initial Guidance: Management of Infants Born to Mothers with COVID-19" addressing the care of infants whose mothers have suspected or confirmed coronavirus disease 2019 (COVID-19). Key points: |
| - Current evidence is consistent with low rates of peripartum transmission and is inconclusive about in utero transmission of SARS-CoV-2 from mothers with COVID-19 to their newborns. -Neonates can acquire SARS-CoV-2 after birth. Their immature immune system leaves newborns vulnerable to other serious respiratory viral infections, raising concern that SARS-CoV-2 may cause severe disease among neonates. -Airborne, Droplet, and Contact Precautions should be utilized when attending deliveries from women with COVID-19 due to the increased likelihood of maternal virus aerosols and the potential need to administer newborn resuscitation to infants with COVID-19 infection that can generate virus aerosol. -When the physical environment allows, newborns should be separated at birth from mothers with COVID-19. -SARS-CoV-2 has not been detected in breast milk to date. Mothers with COVID-19 can express breast milk to be fed to their infants by uninfected caregivers until specific maternal criteria are met. -Infants born to mothers with COVID-19 should be tested for SARS-CoV-2 at 24 hours and, if still in the birth facility, at 48 hours after birth. Centers with limited resources for testing may make individual risk/benefit decisions regarding testing. Note that there have been reports of neonates who test negative at 24 hours but positive at 48-72 hours. -A newborn who has a documented SARS-CoV-2 infection (or who remains at risk for postnatal acquisition of COVID-19 due to inability to test the infant) requires frequent outpatient follow-up via telephone, telemedicine, or in-person assessments through 14 days after discharge. -After hospital discharge, a mother with COVID-19 is advised to maintain a distance of at least 6 feet from the newborn, and when in closer proximity use a mask and hand-hygiene for newborn care until (a) she is afebrile for 72 hours without use of antipyretics, and (b) at least 7 days have passed since symptoms first appeared. -A mother with COVID-19 whose newborn requires ongoing hospital care should maintain separation until (a) she is afebrile for 72 hours without use of antipyretics, and (b) her respiratory symptoms are improved,and (c) negative results are obtained from at least two consecutive SARS-CoV-2 nasopharyngeal swab tests collected ?24 hours apart. These recommendations are based on the most conservative CDC recommendations for discontinuing transmission-based precautions for patients with COVID-19 in the hospital setting and are more stringent than the requirements for mothers and well newborns after hospital discharge. For detailed information on the 2019 Novel Coronavirus (COVID-2019) outbreak, please visit the 2019 Novel Coronavirus (COVID-19) entry in the Red Book Online Outbreaks section. |
Children and young adults may not be as likely to get severely ill or die from COVID-19 as older adults, but experts say they have an important role in stopping its spread and keeping those who are most vulnerable from getting sick.
The predominant signs and symptoms of COVID-19 reported to date among all patients are similar to other viral respiratory infections. These include fever, cough, and difficulty breathing. Gastrointestinal symptoms, including abdominal pain, diarrhea, nausea, and vomiting, were reported in a minority of adult patients. It is important for pediatric providers to have appropriate suspicion of COVID-19, but also to continue to consider and test for other diagnoses, such as influenza.
Children’s Medical Services has approved the use of Skype, Facebook messenger, Face time and What’s App as examples to communicate with patients. See attached PDFs. Baptist is working on policies to allow the use of one Physical Exam for the patient so as to decrease the need for consultants to don PPE to actually lay on hands. PDF instructions attached. COVID19 results that were sent to Mayo or the DOH are scanned into the system. If you click on the “see scanned report” a box will pop up. There is a button that says image. If you click on that….the report is there. The Labcorp results will directly interface but they are taking 5-9 days currently.
Download Removal Hotsheets for:
The scanned reports that we used to find in the ClinDoc tab now appear directly in the results review section. The COVID19 results that come back fastest are the ones from DOH and from Mayo. Those get scanned into results review now. There is a box in the results that says “see scanned report”. If you click on it a box pops up and in the middle of the bottom there is an image button.
The image is the scanned report.
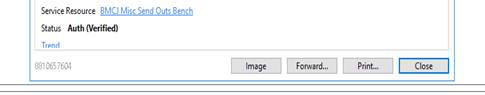
If the result is back (it says see scanned results in the box) and you can’t find the image button, you can find the image by going to the results tab from the actual order. There are 2 lines in the result section. One of them actually has the result. You can also tell looking at the order whether the test went to Labcorp or the DOH/Mayo. In this example the DOH order was cancelled (ie the DOH said they would not run it) so it was sent to Labcorp and has since been completed. If you click on a completed order it takes you to a box that has all of the order information including the results.

For assistance on this, please contact Deborah Abram, Chief Medical Information Officer Pediatric Hospitalist, Physician Quality Lead, Wolfson Children’s Hospital at 904.226.1240 or deborah.abram@bmcjax.com .
When triaging patients, determine the most appropriate venue for care (ex, telemedicine visit, office visit, ER).
Use clinical judgment in determining if diagnostic testing is necessary and rule out other potential causes of respiratory illness (ex., influenza). Consider the following:
- Local epidemiology of COVID-19
- Clinical course of illness, including the presence of symptoms (fever, cough, shortness of breath)
- History of travel to an affected geographic area within 14 days of symptom onset
- Close contact with laboratory confirmed COVID-19 patient within 14 days of symptom onset
If you believe diagnostic testing for COVID-19 may be warranted, work with your local and state public health department to test patients for COVID-19 through approved public health laboratories.
Clinicians should use their judgment to determine if a patient has signs and symptoms compatible with COVID-19 and whether the patient should be tested. Most patients with confirmed COVID-19 have developed a fever and/or symptoms of acute respiratory illness (e.g. cough, difficulty breathing). Some people can become infected, but do not develop any symptoms and/or not feel unwell. Additionally, some individuals have recovered from COVID-19 without needing special treatment, and recover with social distancing and self-quarantine recommendations.
Priorities for testing may include:
- Hospital patients who have signs and symptoms compatible with COVID-19 in order to inform decisions related to infection control.
- Other symptomatic individuals such as, older adults (ages 65 or older) and individuals with chronic medical conditions and/or an immunocompromised state that may put them at higher risk for poor outcomes (e.g. diabetes, heart disease, receiving immunosuppressive medications, chronic lung disease, chronic kidney disease).
- Any persons, including healthcare personnel, who within 14 days of any symptom onset had close contact with a suspect or laboratory-confirmed COVID-19 patient, or who have a history of travel from affected geographic areas within 14 days of their symptom onset.
There are epidemiologic factors that may also help guide decisions about COVID-19 testing. Documented COVID-19 infections in a jurisdiction and known community transmissions may contribute to an epidemiologic risk assessment to inform testing decisions. Clinicians are strongly encouraged to test for other causes of respiratory illness (e.g., influenza).
Mildly ill patients should be encouraged to stay home and contact their healthcare provider by phone for guidance and clinical management Patients who have severe symptoms, such as difficulty breathing, should seek care immediately. Older patients and individuals who have underlying medical conditions or are immunocompromised should contact their physician early in the course of even mild illness.
Source: American Academy of Pediatrics
You can prepare to handle suspected cases in your patient population in the same way you prepare for other respiratory infection outbreaks, such as influenza or RSV. The same principles apply:
- Keep children out of the health care system if it’s not necessary
- Use telemedicine and other non-direct care, when appropriate
- Review infection-control measures, including asking patients with symptoms to call ahead so they can be evaluation in isolation from other patients
- Visual alerts to inform staff of symptoms upon registration and reminders about respiratory hygiene and cough etiquette. Collaborate with hospitals and health systems on local response and to prepare for surges
- Check with local and state health departments for information on specific local and state responses
Source: American Academy of Pediatrics
Child Screening:
If a child has mild symptoms of COVID-19, including fever, cough, or trouble breathing, we recommend parents/guardians call the pediatrician’s office during normal business hours or the Florida Department of Health’s hotline at 866-779-6121 for advice BEFORE heading to the doctor’s office or Emergency Center.
After hours and on weekends, if a child is having mild symptoms, we urge parents/guardians:
Contact a child’s pediatrician to reach the after-hours assistance line (if applicable). OR
Complete a virtual visit via Telescope Health. Staffed by emergency medicine physicians, Telescope Health (www.telescopehealth.com) is open 7 days a week, 6am – midnight. Insurance is not required.
For COVID-19 screening:
1. Duval County Residents - $25 with promo code HERE4YOU and the City of Jacksonville will reimburse at a later date.
2. Non-Duval County Residents - $25 with promo code HERE4YOU.
All non-COVID-19 Telescope Health visits cost $49.
If a child is experiencing severe symptoms, refer the child to the nearest Emergency Center and call ahead to inform the clinicians. Health care providers should notify both infection control personnel at their health care facility and their County Health Department if they identify a person under investigation (PUI) for COVID-19.
NCOV Provider Algorithm
Clinical Screening Provider Algorithm for Identifying Persons Under Investigation for Coronavirus Disease 2019 (COVID-19) per Centers for
Disease Control and Prevention (PDF)
Screening Form
Coronavirus Disease 2019 (COVID-19) Interim Person Screening Form (PDF)
Individuals are strongly urged to not visit, but call their health care provider or local county health department (CHD) with concerns about COVID-19 if they meet one of the following criteria:
- Experiencing symptoms of fever, cough, and/or shortness of breath. These symptoms may appear 2-14 days after exposure to COVID-19
- Have returned from international travel or a cruise within the last 14 days and have the symptoms listed above
- Individuals has closely been around someone with a confirmed diagnosis of COVID-19
Local Florida Department of Health Contact Information
| Baker County | (904) 259-6291 |
| Clay County | (904) 529-2800 or (904) 272-3177 |
| Duval County | (904) 253-2276 |
| Nassau County | (904) 875-6100 |
| St. Johns County | (904) 209-3250 |
| Florida Department of Health COVID-19 24/7 Hotline | (866) 779-6121 |
COVID 19 Screening and Testing Process Info
Go to Telecscope Health website
For Duval County Residents
-If a pediatric or adult patient lives in Duval County and receives a testing order from Telescope Health, he/she will receive an appointment to go to the drive-thru testing site at the Prime Osborn Convention Center in downtown Jacksonville (1000 Water Street).
-Individuals must complete a screening and receive a physician order through Telescope Health in order to participate. No walk-ups without prior Telescope Health screening will be accepted.
-Location: Prime Osborn Convention Center (1000 Water Street), west parking lot
Federally-Sponsored Testing Site
-Individuals must exhibit respiratory symptoms to be tested. A maximum number of 250 people per day will be tested at this site.
-First responders and healthcare workers who have direct contact with patients will be tested regardless of the presence of symptoms (fever, cough, difficulty breathing)
-Location: TIAA Bank Field, Lot J
Ascension/St. Vincent’s
COVID 19 Screening and Testing Info
Patients must be pre-screened by an Ascension provider before they are directed to the testing site.-Patients may also use the Ascension Online Care website for a screening
-Location: Determined in pre-screening consultation
Wolfson Children's Hospital
Pediatricians should call one of these numbers in advance if you have someone that needs to be tested at WCH Emergency Departments:
Main Campus: 904-202-9000 South: 904-271-6060 Clay: 904-516-1100
Town Center: 904-202-6790 North: 904-202-6891 Oakleaf: 904-516-1620
St. Johns County Testing Sites
https://www.news4jax.com/news/local/2020/03/24/getting-tested-here-are-the-spots-in-st-johns-county/
Florida Regional County Contacts - Download List for 18 Counties
GEORGIA
Evaluation and testing for COVID-19 is not available at public health clinics.
Federal and state agencies are working on a plan to roll out widespread drive-through testing for COVID-19 across the United States. However, all testing for COVID-19 in Georgia at this time must be coordinated by a healthcare provider. You cannot walk into a pharmacy or commercial lab and request a test for yourself.
Typically, testing is reserved for people who are sick with symptoms of COVID-19, people who have been in close contact with a person known to have COVID-19, or people who recently traveled to an area with ongoing spread.
The most common symptoms of COVID-19 are fever, dry cough, and shortness of breath. If you have symptoms of COVID-19, it’s important not to show up at your doctor’s office, urgent care, or emergency room without giving the facility advance notice. If you walk in the front door and are contagious, you could be putting other patients at risk of exposure. Call first and follow their instructions.
If you visit your doctor and a test is ordered, your nose and throat will be swabbed and the swab will be sent off to a laboratory to be tested for the virus.
At this point, all swabs are sent to either the state health lab, or a private lab, like LabCorp. All positive test results are reported to the Georgia Department of Public Health.
Test results can take from 24 hours to several days. There is no rapid test for COVID-19 right now.
The Health Department has testing and is referring individuals to their PCP or other qualified provider for screening.
- Due to testing capacity, the Health Department is only able to test patients meeting certain criteria.
- If the individual meets the state’s criteria, healthcare providers should complete an online testing request at the following link: https://dhpexternal.dph.ga.gov/covid19_test_req.html
-If testing is approved, healthcare providers will receive information with laboratory forms and shipping instructions.
Sites
If you experience fever, cough or shortness of breath call your health care provider
Nemours is following the recommendations of our infectious disease experts and the Centers for Disease Control and Prevention (CDC) and postponing all non-urgent surgery and specialty appointments.
Location Updates for Nemours Children’s Specialty Care, Jacksonville
· Nemours Children’s Specialty Care (downtown) is continuing visits M-F 8 a.m. to 5 p.m. for urgent, in-person visits.
- Nemours Children's Specialty Care, Fleming Island is closed.
- Nemours Children’s Specialty Care, Jacksonville South is closed.
- Telehealth Option: 24/7 Online Doctor Visits with Nemours CareConnect:
- Nemours is always here to care for kids who are sick. If a child doesn’t feel well, the child and parent can have a telehealth visit with Nemours CareConnect to find out what to do.
- Parents can download Nemours CareConnect from the App Store or Google Play.
- Nemours CareConnect features:
• No appointment needed
• Board-certified Nemours experts
• Available 24/7/365
• Private and secure, HIPAA compliant connection
• Cost $0 – $59 per visit, depending on insurance benefits
For Nemours Children’s Specialty Care In-person Visits:
· Only one healthy parent or caregiver can accompany a child to a specialty care visit. Siblings and other family or friends may not be at these visits.
· If you are feeling sick, find a healthy caregiver to bring your child to their scheduled appointment. If you can’t find a healthy adult to accompany your child, please call Nemours to reschedule your appointment.
· NOTE: These rules only apply at Nemours locations. Policies at our affiliated hospitals may be different. The check-in staff at those locations will explain their policy.
| Centers for Disease Control and Prevention | Information for Pediatric Healthcare Providers https://www.cdc.gov/coronavirus/2019-nCoV/hcp/pediatric-hcp.html Update Guidance on Evaluating and Testing Persons for Coronavirus Disease 2019 (COVID-19) http://emergency.cdc.gov/han/2020/han00429.asp Interim Infection Prevention and Control Recommendations for patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings https://www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html Pregnancy & Breastfeeding: Information about Coronavirus Disease 2019 https://www.cdc.gov/coronavirus/2019-ncov/prepare/pregnancy-breastfeeding.html |
| American Academy of Pediatrics | Critical Updates on COVID-19 https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/ COIV-19 Pandemic – Clinical Guidance for Pediatric Patients https://www.youtube.com/watch?v=9krOSPYrgH8 |
| Florida Department of Health | https://floridahealthcovid19.gov/ For Healthcare Providers https://floridahealthcovid19.gov/health-care-providers/ Duval County http://duval.floridahealth.gov/ St. Johns County http://stjohns.floridahealth.gov/ Clay County http://clay.floridahealth.gov/ Nassau County http://nassau.floridahealth.gov/ Baker County http://baker.floridahealth.gov/ |
| Wolfson Children’s Hospital | COVID-19 and Children https://www.wolfsonchildrens.com/covid19 |
Supplemental Fact Sheet Addressing the Risk of COVID-19 in Preschool, Elementary and Secondary Schools While Serving Children with Disabilities(PDF) – from the US Department of Education. A clarification about distance learning to amend an earlier policy statement. Notes that ensuring compliance with the Individuals with Disabilities Education Act (IDEA) and other federal regulations should not prevent any school from offering educational programs through distance instruction.
Child Rights International Network (CRIN.ORG) – Insightful information for professionals and helpful tools including “imagination kits” for families who are at home with children.
APPLE CDC app available for iPhones: https://apps.apple.com/us/app/id1504132184
HELPFUL INFO FOR FAMILIES WITH CHILDREN Great information for families talking with their children, caring for themselves and their children and learning through Mr. Rogers at the Fred Rogers Center https://www.fredrogerscenter.org/what-we-do/child-wellness/coronavirus-response
Send in your questions and comments to our pediatric team.
March 31, the Agency for Health Care Administration and Department of Children and Families announced that Medicaid eligibility will be continued through the last day of the state of emergency.
The agencies are working to identify and inform people who have been terminated in March to know that their Medicaid will be reinstated and continued.
There is also an extension of time to complete Medicaid applications to 120 days, effective February 2020. For example, if someone applied March 7, 2020, they will have until July 4 to submit paperwork, and Medicaid eligibility will be effective retroactively to March 1.
If you have clients who were terminated from Medicaid in March and have not been notified of their restored eligibility, please share that information on this listserv or contact us directly. The Florida Policy Institute (FPI) and Florida Health Justice Project (FHJP) are working with DCF and AHCA on COVID related policies and issues and will follow-up.
A new website for COVID-19 http://ahca.myflorida.com/covid-19_alerts.shtml has a number of policy transmittals which include direction to both fee-for-service and managed care plan providers. Significantly, the introduction to Policy Transmittal 2020-15 states that during the state of emergency, " managed care plans must ensure there are no gaps in care for its Medicaid enrollees."
Important new requirements during the emergency that are applicable to all of Florida's Medicaid managed care plans, including long-term care (LTC) plans (as well as fee-for service) include:
Waiver of prior authorization requirements for the following services:
- Skilled nursing facilities
- Long term acute hospitals
- Hospital services
- Physician services
- Advanced practice registered nursing
- Physician assistant
- Home health
- Durable medical equipment and supplies
Services for patients diagnosed with COVID-19
Plans must waive limits on medically necessary services (specifically duration, frequency and scope). For example, the 45 day limit on hospital services will not apply.
Prescription drugs:
While prescriptions drugs are still subject to prior authorization requirements, plans must lift all limits on early refills (except for controlled substances). Also, coverage will be provided for 90 day supplies, when requested by the enrollee. ( Emphasis added. )
Copayments: must be waived
Appeals and fair hearings:
Enrollees must be given more time to appeal through their plan or request a fair hearing if the need for an extension is due to COVID-19 impact. The policy transmittal also notes that while the Agency got permission from the federal government to delay scheduling of Medicaid fair hearings and issuing decisions (this was through the 1135 waiver granted last week and referenced in Val Greenfield's 3/20 message to the health/senior listserve), hearing delay is limited to those instances where the recipient is continuing to receive services pending the outcome of the fair hearing. (emphasis added.)
Telemedicine:
AHCA is encouraging maximized delivery of services via telemedicine and has several provider specific transmittals on the website to give providers guidance.
Expansion of LTC Provider qualifications:
Given the impact COVID-19 is having on network providers "resulting in potential closures and workforce shortages," the Agency is authorizing plans to temporarily modify services and provider qualifications during the state of emergency. The plan must ensure that the provider use appropriately licensed staff to perform services within the individual's scope of practice, and the plan must document the reason for the temporary alteration in the plan of care.
Postponement of preadmission screening and residence reviews for nursing homes
Free public benefits application assistance for Medicaid, SNAP, TCA.
Call 904-202-5001 to get help with the application.
Download Public Benefits Application Assistance info
SNAP, TANF and MEDICAID - an extremely helpful summary of recent changes to FL’s Safety Net policies due to the COVID-19 crisis. The summary was created by Florida Policy Institute.
Download DCF Transmittal for SNAC newsletter
- Suspension of work requirements for SNAP and TANF
- A SNAP additional, emergency allotment
- Suspension of Medicaid terminations
- Extension of April & May recertifications for DCF ACCESS programs
As Florida’s response to COVID-19 takes front and center, concern grows for low-income families who struggle to take precautions again the spread of the virus. Although Congress has passed the Families First Coronavirus Response Act to address, at least in part, the public health crisis and economic fallout from COVID-19, many barriers continue to keep struggling families from accessing the assistance they need during the pandemic. As Florida initiates policies implementing the Act, FPI will keep you updated. In return, we ask that you please keep us apprised of systemic issues impacting families applying for or receiving safety new program assistance.
SNAP, TANF, AND MEDICAID
- DCF closes offices. DCF announced on March 18, 2020, that it was closing brick-and-mortar storefronts due to coronavirus. Because 10 percent of persons apply for safety net benefits in person, DCF recommends that those Floridians use drop-boxes at the Department’s storefront locations to turn in their applications. Although families can complete interviews telephonically due to office closures, applications are not accepted by phone At the same time, seniors and persons with significant medical conditions, many of whom do not have internet access or a computer, have been advised by health and governmental authorities to stay home. It is unclear whether DCF is developing a plan to serve those Floridians who may wish to apply for assistance during the pandemic.
- DCF expands call center hours. DCF has expanded call center hours from 7 a.m. to 6 p.m. (EST), Monday through Friday. Call center numbers are 866-762-2237 or 850-300-4DCF.
- Certification periods extended by 6 months.Certification periods for cash, food and medical assistance have has been extended by 6 months for individuals and families scheduled to recertify in April or May 202. As of now, persons who were due to recertify in March must still recertify over the internet or fill out a hard copy recertification application. However, individuals and families eligible for Medicaid in March do not have to recertify and their coverage should be reinstated if it was terminated during March.
- DCF switches to phone interviews.Phone interviews are now being used for cash and food assistance.
- Mandatory work requirements suspended effective immediately.Governor DeSantis directed DCF to waive work requirements for SNAP and TANF. In response, DCF says that, effective immediately, it will waive work requirements for individuals participating in the Supplemental Nutrition Assistance Program (SNAP) and Temporary Assistance for Needy Families (TANF) program. To do this, DCF explains that it has partnered with the Department of Economic Opportunity to apply good cause statewide for TANF and SNAP recipients who would otherwise be subject to participate in mandatory work requirements as a condition of receiving those benefits.
- DCF makes adjustments to reflect 2020 Federal Poverty Level (FPL).DCF has issued the attached transmittal governing the 2020 Federal Poverty Level (FPL) and Consolidated Need Standards (CNS) to increase the income limits for the Medicaid and Temporary Cash Assistance (TCA) Programs. DCF says that the new adjustments will be used in the determination of eligibility for pending applications and open cases effective April 1, 2020.
SNAP
- Emergency allotments to be issued.DCF has been directed by Governor DeSantis to automatically supplement SNAP allotments of current recipients up to the maximum for families of the household’s size for 2 months. According to the waiver request submitted by DCF to USDA to make this allotment, DCF says that March supplements will be added on April 4, 2020, and that April supplements will be issued either on April 8th or on a family’s regular staggered issuance date.
- Pandemic EBT directed by Governor. Governor DeSantis has directed DCF to provide SNAP to families whose children are eligible for free and reduced-price school lunch. Although the devil is in the details, Florida expects to cover more than 2.1 million children in Florida.
- What about Disaster SNAP (D-SNAP)?Although a COVID-19 disaster declaration been declared both at the federal and state level, no D-SNAP program has been approved by the President or initiated as of now. Disaster Supplemental Nutrition Assistance Program (D-SNAP) is linked to a federal declaration of disaster approving the program.
- Time limit suspended. The three-month time limit on SNAP eligibility for ABAWDS is suspended effective April 1, 2020, until the public health emergency is lifted. DCF has not yet issued details about implementation of this suspension.
SCHOOL MEALS
- Florida’s Agriculture Commissioner takes early steps to feed school children during school closures. Many of Florida's low-income children will still be able to get free and reduced-cost meals even though their schools are closed because of the pandemic. Families can locate free meals for children under 18 through the Florida Department of Agriculture and Consumer Services’ (FDACS) Summer BreakSpot website. For most sites, no application is required.
REEMPLOYMENT ASSISTANCE (RA)
- RA work-search and work registration requirements waived. Persons filing an application for RA benefits from March 15, 2020 until May 2, 2020, will not be required to complete the work registration in Employ Florida. In addition, the work search requirement for individuals requesting benefits for the weeks of March 15, 2020 to May 2, 2020 is also waived.
- What aboutPandemic Unemployment Compensation, Pandemic Emergency Unemployment Compensation, and Pandemic Unemployment Assistance? Help is on the way from Congress to add on an additional $600 per week in unemployment compensation for RA and Pandemic Unemployment Assistance claimants as well as to provide additional weeks of assistance. Here is a link to the National Employment Law Project’s analysis of UI provisions in the CARES Act.
MEDICAID
- New Policy: No Medicaid terminations from March through the end of the public health emergency. On March 31st, AHCA alerted providers that:
- No Medicaid recipient will lose Medicaid eligibility during the COVID-19 public health emergency;
- AHCA is working to notify recipients who may have received a termination notice in the month of March that their benefits will be reinstated; and
- Effective with applications filed in February 2020, the time for submitting documentation required to process an application is extended for 120 days from the date of the application and eligibility will still be effective the first day of the month the application was received.
Under the newly enacted Families First Coronavirus Response Act, a state is prohibited from ending coverage for recipients enrolled as of March 18th for the duration of the public health emergency if the state opts to obtain an enhanced federal Medicaid match. Florida has opted to take the enhanced match.
St. Jude Coronavirus Activity Book

COVID-19 Fact Sheet in 18 Languages
Hesperian Health Guides has created a Coronavirus (COVID-19) Fact Sheet to share essential information about how the virus infects and spreads, who is most likely to be infected, and how to prevent infection. The fact sheet is available in 18 languages! Here is the full list:Arabic, Bangla, Chinese, English, Farsi, Filipino, French, Haitian Creole, Hindi, Indonesian, Portuguese, Sindhi, Shona, Swahili,Telugu, Spanish, Urdu, and Vietnamese. We encourage you to share Hesperian’s fact sheet widely to ensure that everyone, everywhere has the critical information to protect their health.
AAP News features the latest articles, research and news from the American Academy of Pediatrics view website
AAP Communications: Pediatric practices will receive funding through in round of funding through CARES Act
https://www.aappublications.org/news/2020/04/09/caresact040920
AAP COVID-19 Communications/Resources and Updates for Pediatricians (April 2020)
The American Academy of Pediatrics (AAP) has recorded the following 20 minutes COVID-19 webinars:
- Coding During the COVID-19 Pandemic: Navigate rapidly evolving ICD-10-CM and CPT coding for telemedicine, telehealth, and COVID-19 diagnoses and procedures during this unprecedented time.
- COVID-19 Pandemic – Clinical Guidance for Pediatric Practices: Strategies for pediatricians to increase infection control practices in their office to protect patients and health care workers.
- Talking to and Supporting Children During a Pandemic: Helping clinicians understand how to support children during the COVID-19 pandemic, including helping parents talk to their children.
- Disaster Management for the Pediatrician in the COVID-19 Response: Utilizing preparedness principles to mobilize pediatricians to adapt protocols, learn new models, and serve as champions for children in a pandemic.
- Caring for Children with Complex Medical Conditions During COVID-19: Helping clinicians understand special considerations for children with complex needs, how to prepare the clinic/practice, and how to partner with and support families.
- Telehealth and COVID-19: Offering tips and support for how to launch telehealth into practice.
The AAP has a main COVID-19 web page that highlights the latest AAP resources, guidance, recommendations from the Centers for Disease Control and Prevention (CDC), information to share with parents, and other materials. This page is updated daily as new information is available. Links to specific AAP resources follow:
- AAP News: Coronavirus Disease Outbreak Coverage
- AAP Red Book Online, Outbreaks – COVID-19: 2019 Novel COVID-19 Infections
- AAP Disaster Preparedness Information: Preparedness Checklist for Pediatric Practices and Pediatric Readiness in the Emergency Department
- Additional AAP Guidance
- Ways to Keep Children Occupied During These Challenging Times
- Advises Parents Experiencing Stress over COVID-19
- Telehealth Payer Policy in Response to COVID-19
- Coding for COVID-19 and Non-Direct Care
- State Notices on Telehealth Policy in Response to COVID-19
- Telehealth Care and After Hours Care
- HealthyChildren.org: Working and Learning From Home During the Covid-19 Outbreak
- HealthyChildren.org: Positive Parenting & COVID-19: 10 Tips to Help Keep the Calm at Home
- HealthyChildren.org: Tips for Coping with a New Baby During COVID-19
- HealthyChildren.org: Social Distancing: Why Keeping Your Distance Helps Keep Others Safe
- HealthyChildren.org: Positive Parenting & COVID-19: 10 Tips to Help Keep the Calm at Home
- HealthyChildren.org: Coping with a New Baby During COVID-19
- HealthyChildren.org: Hand Washing: A Powerful Antidote to Illness
- HealthyChildren.org: Talking to Children About Tragedies & Other News Events
- HealthyChildren.org: Ask the Pediatrician: Are there shortages of infant formula due to COVID-19?
- HealthyChildren.org: COVID-19: Information for Families of CYSHCN
The Families First Coronavirus Response Act (FFCRA or Act) requires certain employers to provide employees with paid sick leave or expanded family and medical leave for specified reasons related to COVID-19. The Department of Labor’s (Department) Wage and Hour Division (WHD) administers and enforces the new law’s paid leave requirements. These provisions will apply from April 1, 2020 through December 31, 2020.
Plan, Prepare, and Respond – Interim guidance for businesses and employers responding to Coronavirus, May 2020 https://www.cdc.gov/coronavirus/2019-ncov/community/guidance-business-response.html
From the Florida Department of Health, the Bureau of Personnel and Human Resource Management has drafted the Returning from Telework Plan to help guide the Department in systematically bringing employees back to the workplace as Florida begins to re-open. View PDF
To get and keep America open must be able to act quickly to identify new cases, break chains in transmission and protect first responders and health care workers.
Here’s the latest from the CDC. https://www.cdc.gov/coronavirus/2019-ncov/php/open-america/index.html?deliveryName=USCDC_2067-DM26308
The Florida Chapter of the American Academy of Pediatrics, in an 11-page white paper dated July 28, sent to Gov. Ron DeSantis, pointed to “significant benefits” of children going back to school but also said those benefits have to be weighed against the risks. In the report, FCAAP outlines thirteen in-depth recommendations for how to carefully and effectively reopen schools in Florida.
Children Need Vaccines To Start SchoolImmunizations Press Release
Back to School Immunizations Flyer